Last updated on September 15, 2022
A Dallas anesthesiologist who allegedly injected nerve blocking agents and other drugs into patient IV bags at a local surgery center – resulting in at least one death and multiple cardiac emergencies – was arrested Wednesday on federal criminal charges, Principal Deputy Assistant Attorney General Brian M. Boynton, head of the Justice Department’s Civil Division, and U.S. Attorney for the Northern District of Texas Chad E. Meacham announced today.
Raynaldo Rivera Ortiz Jr., 59, was charged via criminal complaint with tampering with a consumer product causing death and intentional drug adulteration. He was arrested in Plano on Wednesday and will make his initial appearance before U.S. Magistrate Judge Renee Toliver in Dallas on Friday at 10 a.m.
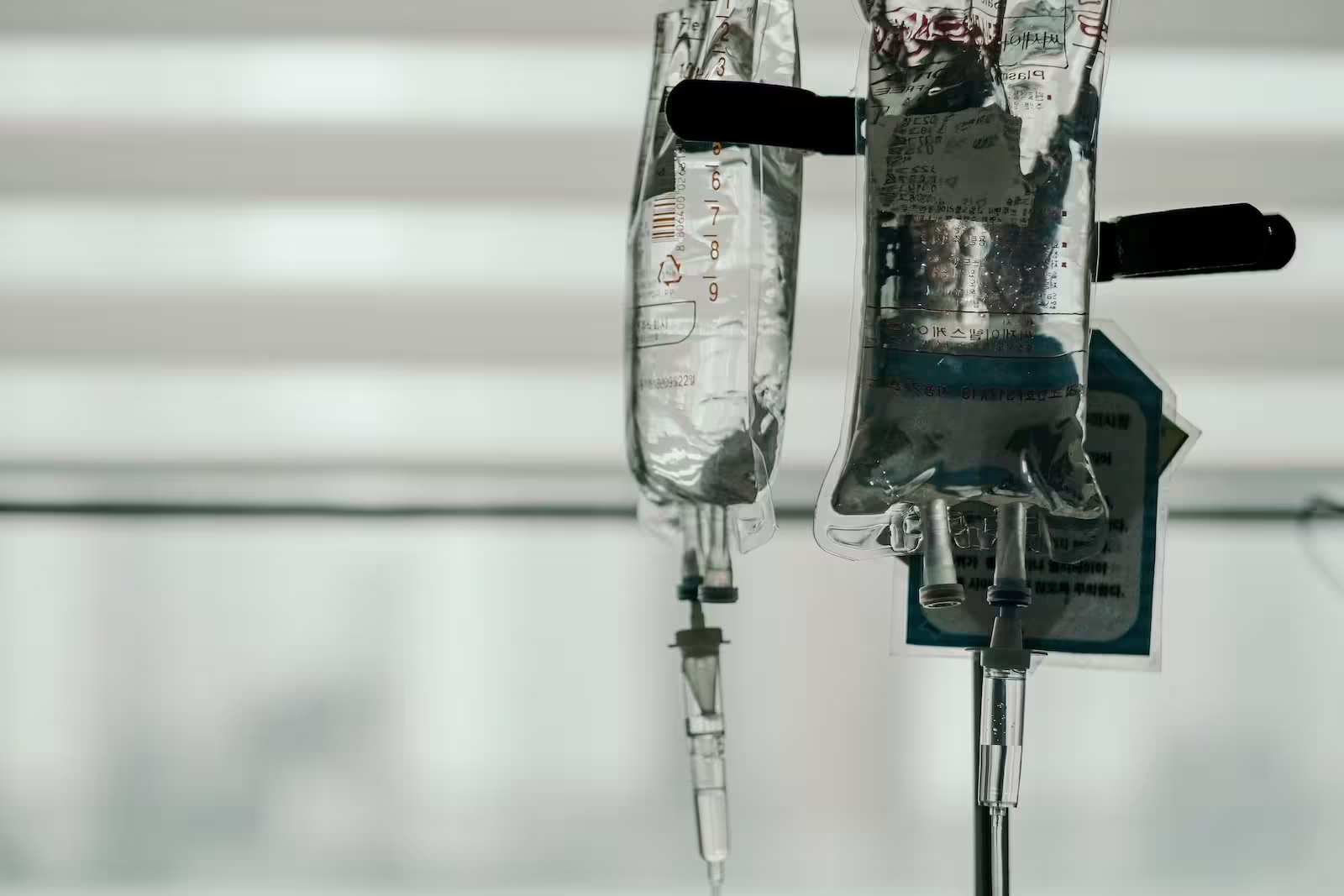
According the criminal complaint, on June 21, a 55-year-old female coworker of Dr. Ortiz, identified in court documents as M.K., experienced a medical emergency and died immediately after treating herself for dehydration using an IV bag of saline taken from the surgical center. An autopsy report revealed that she died from a lethal dose of bupivacaine, a nerve blocking agent that is rarely abused but is often used during the administration of anesthesia.
Two months later, on Aug. 24, an 18-year-old male patient, identified in court documents as J.A., experienced a cardiac emergency during a routine sinus surgery. The teen was intubated and transferred to a local ICU. Chemical analysis of the fluid from a saline bag used during his surgery revealed the presence of bupivacaine, epinephrine (a stimulant), and lidocaine, drugs that could have caused the patient’s sudden symptoms.
According to the complaint, surgical center personnel concluded that the incidents involving M.K. and J.A. suggested a pattern of intentional adulteration of IV bags used at the surgical center. They identified 10 additional unexpected cardiac emergencies that occurred during otherwise unremarkable surgeries between May and August 2022, which the complaint alleges is an exceptionally high rate of complications over such a short period of time.
In each of those cases – which investigators believe occurred on or around May 26 and 27; June 27; July 7, 15 and 18; and Aug. 1, 4, 9 and 19 – medical personnel were able to stabilize the patient only through use of emergency measures. Most of the incidents occurred during longer surgeries that used more than one IV bag, including one or more bags retrieved mid-surgery from a stainless steel bag warmer.
The complaint alleges that none of the cardiac incidents occurred during Dr. Ortiz’s surgeries, and that they began just two days after Dr. Oritz was notified of a disciplinary inquiry stemming from an incident during which he allegedly “deviated from the standard of care” during an anesthesia procedure when a patient experienced a medical emergency. The complaint alleges that all of the incidents occurred around the time Dr. Ortiz performed services at the facility, and no incidents occurred while Dr. Ortiz was on vacation.
The complaint further alleges that Dr. Ortiz, who had a history of disciplinary actions against him, expressed concern to other physicians over the disciplinary action at the facility and complained the center was trying to “crucify” him. A nurse who worked on one of Dr. Ortiz’s surgeries allegedly told law enforcement that Dr. Ortiz refused to use an IV bag she retrieved from the warmer, physically waving the bag off.
Surveillance video from the center’s operating room hallway allegedly showed Dr. Ortiz placing IV bags into the stainless-steel bag warmer shortly before other doctors’ patients experienced cardiac emergencies.
The complaint alleges that in one instance captured in the surveillance video, agents observed Dr. Ortiz walk quickly from an operating room to the bag warmer, place a single IV bag inside, visually scan the empty hallway, and quickly walk away. Just over an hour later, according to the complaint, a 56-year-old woman suffered a cardiac emergency during a scheduled cosmetic surgery after a bag from the warmer was used during her procedure.
The complaint alleges that in another instance, agents observed Dr. Ortiz exit his operating room carrying an IV bag concealed in what appeared to be a paper folder, swap the bag with another bag from the warmer, and walk away. Roughly half an hour later, a 54-year-old woman suffered a cardiac emergency during a scheduled cosmetic surgery after a bag from the warmer was used during her procedure.
“The safety of the nation’s pharmaceutical supply is critically important,” said Principal Deputy Assistant Attorney General Brian M. Boynton. “The Department will vigorously prosecute this case consistent with the evidence gathered by our law enforcement partners.”
“Our complaint alleges this defendant surreptitiously injected heart-stopping drugs into patient IV bags, decimating the Hippocratic oath,” said U.S. Attorney Chad E. Meacham. “A single incident of seemingly intentional patient harm would be disconcerting; multiple incidents are truly disturbing. At this point, however, we believe that the problem is limited to one individual, who is currently behind bars. The Department of Justice and our indefatigable partners at the FDA’s Office of Criminal Investigations and the Dallas Police Department will work tirelessly to hold him accountable. In the meantime, it is safe to undergo anesthesia in Dallas.”
“Patients expect that their doctors will use only safe and effective medical products during their surgeries. When illicit tampering occurs, serious harm and even death can result,” said Special Agent in Charge Charles L. Grinstead, FDA Office of Criminal Investigations. “Working with our law enforcement partners, we will continue to monitor, investigate and bring to justice those who would risk patients’ health and safety.”
A criminal complaint is merely an allegation of criminal conduct, not evidence. Dr. Ortiz is presumed innocent unless and until proven guilty beyond a reasonable doubt in a court of law.
If convicted, he faces a maximum penalty of life in prison. A federal district court judge will determine any sentence after considering the U.S. Sentencing Guidelines and other statutory factors.
The U.S. Food & Drug Administration’s Office of Criminal Investigations and the Dallas Police Department conducted the investigation with the assistance of scientists from the University of North Texas. Senior Litigation Counsel Patrick Runkle and Senior Trial Counsel Yolanda McCray Jones of the Justice Department’s Consumer Protection Branch and Assistant U.S. Attorneys John de la Garza and Errin Martin of the Northern District of Texas are prosecuting the case.