Bahar Aliakbarian, Michigan State University
Bahar Aliakbarian is an expert in supply chain management in pharmaceuticals and a professor at the School of Packaging at Michigan State University. Below, she describes the vaccine supply chains of Pfizer and Moderna, which are expected to be the two major early suppliers of the COVID-19 vaccines in the U.S. She also talks about challenges in distribution and the work being done to ensure safe and systematic delivery of the vaccines.
What are the main challenges in distributing the newly developed COVID-19 vaccines?
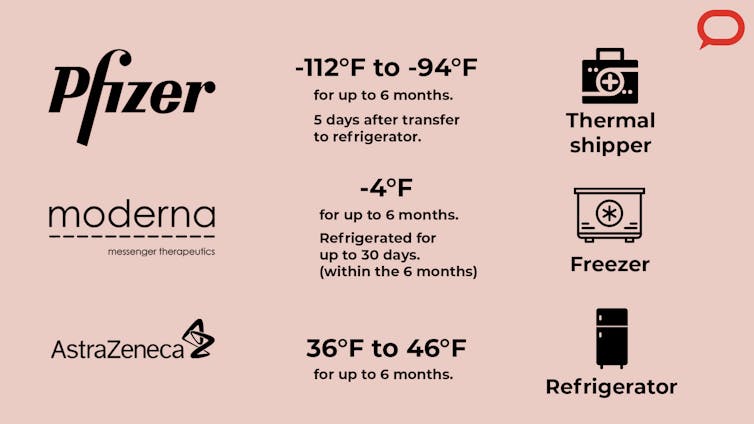
The two major U.S. developers of the early COVID-19 vaccines are Pfizer/BioNTech and Moderna. They both developed mRNA vaccines, a relatively new type of vaccine. A major supply chain issue is the temperature requirement for these vaccines. The Pfizer vaccine needs to be stored at between minus 112 F (minus 80 C) and minus 94 F (minus 70 C), and the Moderna vaccine needs temperatures around minus 4 F (minus 20 C), which is close to the temperature of commercial-grade freezers. A third company developing vaccines, AstraZeneca, says it needs regular refrigeration temperature of 36 F to 46 F, or 2 to 8 C.
Moderna’s vaccine can remain at minus 4 F for up to six months, and then for a month in a refrigerator, according to the company. Pfizer says its vaccine has a shorter shelf life of five days after being transferred from ultracold storage to a refrigerator, leaving a short window to administer the vaccines.
How will these vaccines be transported and stored?
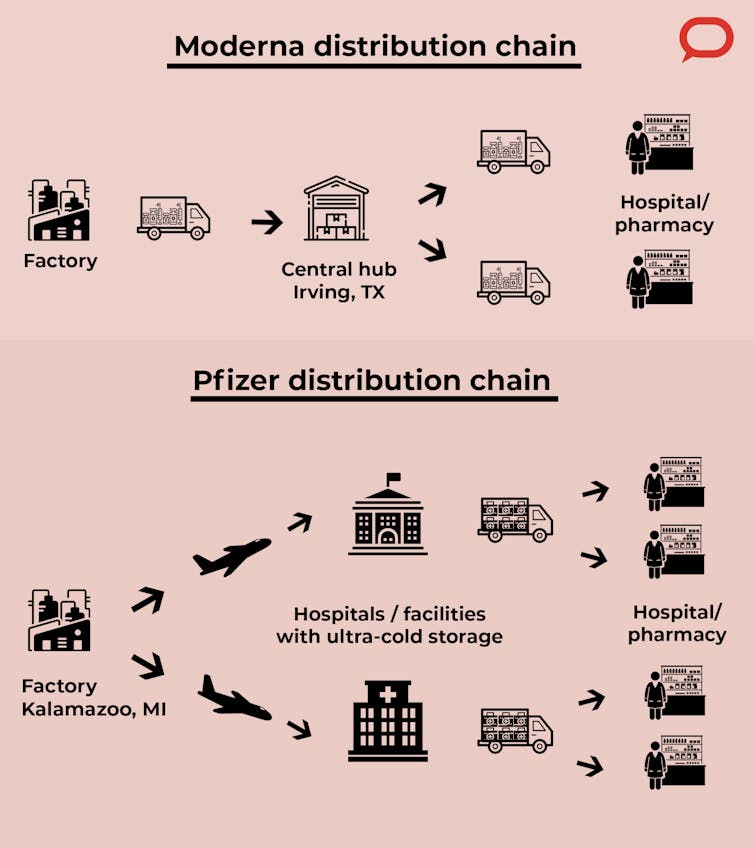
Moderna plans to use an approach similar to that used in previous outbreaks such as the H1N1 swine flu pandemic in 2009. In this case the vaccines will be shipped from the manufacturing facilities in the Northeast U.S. and Europe to a distribution center in Irving, Texas, which will be equipped with freezers to store the vaccine for longer periods. From there they are distributed to hospitals, pharmacies and other vaccine administration sites.
Pfizer is manufacturing its vaccines in Kalamazoo, Michigan. It will handle the transportation to the administration sites by working with logistics partners. Because ultracold storage is available only at large facilities and hospitals, that’s where they’ll be stored for short periods before being distributed to administration sites.
Some states, like New York, are considering setting up their own distribution hubs.
How will the required temperatures be maintained?
Pharmacies and hospitals are trying to develop or acquire ultralow-temperature freezers, but it is a huge cost for them. We’re now seeing extremely high demand for freezers and dry ice, and there is risk of shortage. So the vaccines need to be supplied and administered efficiently to ensure they reach the public without any waste or bottlenecks in the supply chain. In 2019 alone, around US$34 billion worth of vaccines were wasted because of fluctuations in temperature during transportation.
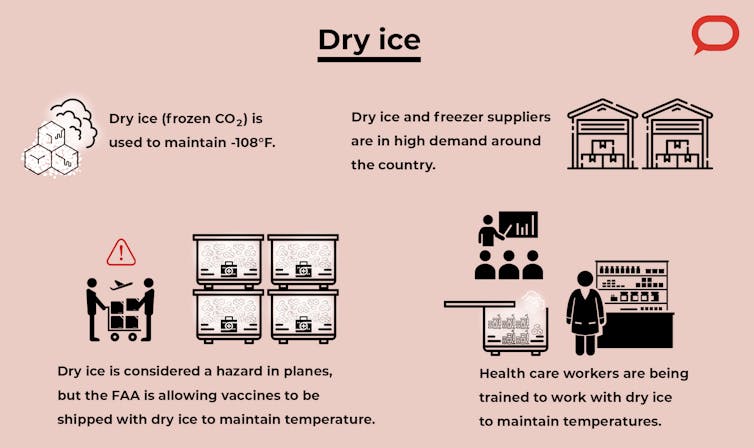
Dry ice is an inexpensive way to maintain low temperatures. Pfizer’s suitcaselike “thermal shippers” need about 50 pounds of dry ice to keep them at temperature for a few days. It is considered to be a hazardous material in planes, but the Federal Aviation Administration has granted permission to use up to five times the normally permitted amount to be transported along with the vaccines.
The staff at administration sites must be trained to check the temperature and make sure that Pfizer’s thermal box is not being opened more than a few times a day, not more than a few minutes at a time, and to fill it with new dry ice at the right times. Some of this training is already underway.
What can be done about monitoring and traceability?
Tracking and monitoring them throughout the process ensures that the vaccines are stable and not tampered with. Making this data accessible to governments and the public can increase trust in the vaccines. This is especially important because these vaccines require two doses to work, and we need people to come back to get the second one, and to follow up with them for feedback about any possible adverse effects.
My team and I are working on developing technologies to improve tracking and monitoring using smart packaging by implementing sensors and other communication technologies.
[Deep knowledge, daily. Sign up for The Conversation’s newsletter.]
Monitoring and tracking also involves developing databases that integrate data within an end-to-end supply chain, from the manufacturers to the administration sites. Right now, Pfizer and Moderna will have the information until it reaches the administration sites, and the hospitals and pharmacies will have the data about the patients though electronic health records (EHR). So there are some challenges we are still trying to overcome to have an integrated and interoperable system with improved capability to be upgraded and used nationwide.
Insurance companies and the government are thinking about how to provide coverage for the vaccines while the Centers for Disease Control and Prevention issues guidelines to ensure that most of the population receives the vaccine efficiently.
Bahar Aliakbarian, Research associate professor of supply chain management, Michigan State University
This article is republished from The Conversation under a Creative Commons license. Read the original article.