As part of the U.S. Food and Drug Administration’s ongoing commitment to fight the Coronavirus Disease 2019 (COVID-19) pandemic, today the agency issued guidance for immediate implementation to address the urgent and immediate need for blood and blood components.
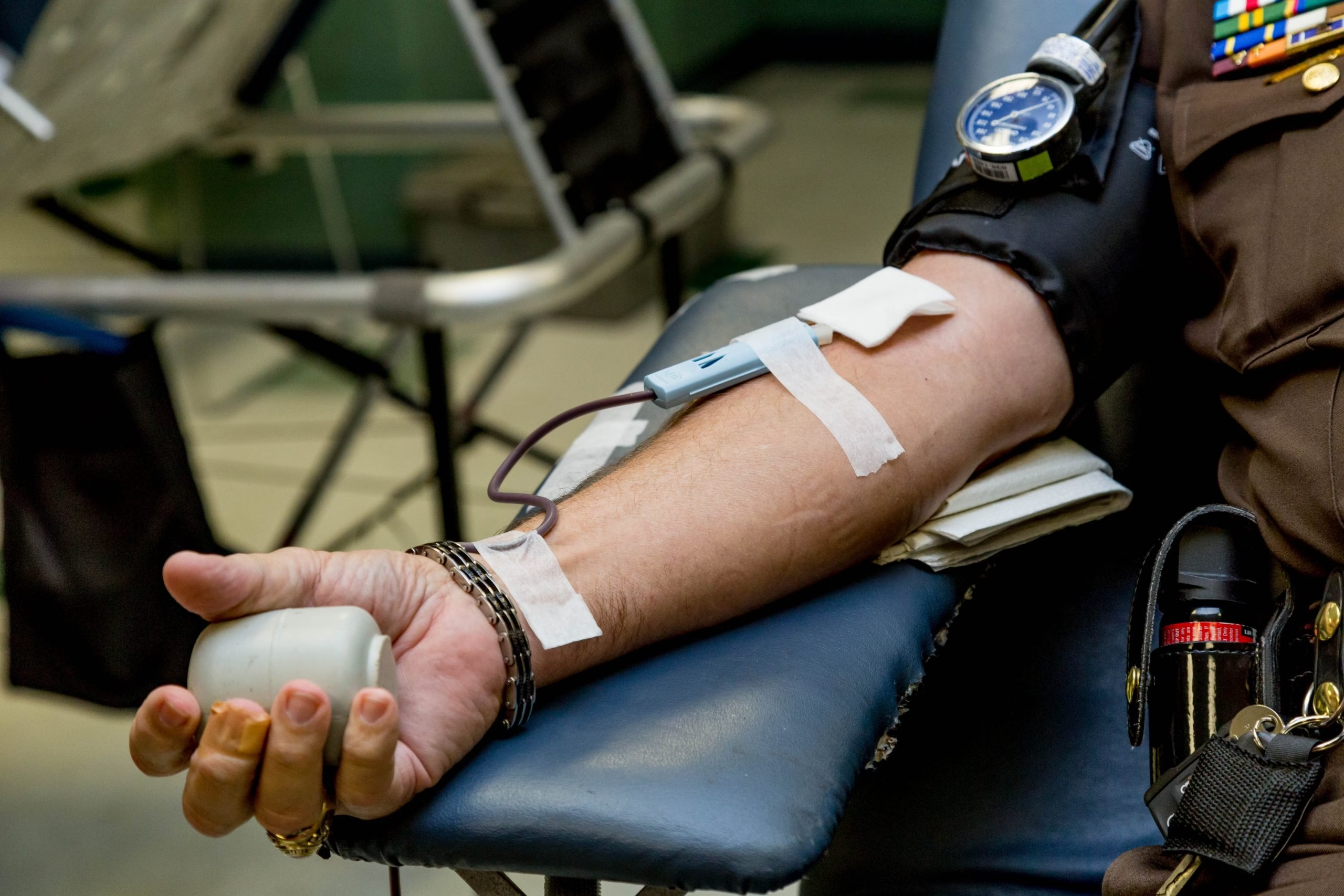
The COVID-19 pandemic has caused unprecedented challenges to the U.S. blood supply. Donor centers have experienced a dramatic reduction in donations due to the implementation of social distancing and the cancellation of blood drives.
Maintaining an adequate blood supply is vital to public health. Blood donors help patients of all ages – accident and burn victims, heart surgery and organ transplant patients and those battling cancer and other life-threatening conditions. The American Red Cross estimates that every two seconds, someone in the U.S. needs blood.
People who donate blood are part of our critical infrastructure industries. More donations are needed at this time and we hope people will continue to take the time to donate blood. We have also encouraged, and continue to encourage, state and local governments to take into account the essential nature of donating blood – and that it can be done safely and consistently within social distancing guidelines – when considering travel and business restrictions, and we encourage them to communicate that to their citizens.
At the FDA, we want to do everything we can to encourage more blood donations, which includes revisiting and updating some of our existing policies to help ensure we have an adequate blood supply, while still protecting the safety of our nation’s blood supply.
Based on recently completed studies and epidemiologic data, the FDA has concluded that current policies regarding certain donor eligibility criteria can be modified without compromising the safety of the blood supply. Therefore, the FDA is revising recommendations in several guidances regarding blood donor eligibility. These changes are being put forth for immediate implementation and are expected to remain in place after the COVID-19 pandemic ends, with any appropriate changes based on comments we receive and our experience implementing the guidances. At this time, the alternatives to certain donor eligibility requirements being provided generally will apply only for the duration of the declared pandemic.
Among others, the FDA is making the following changes, for immediate implementation, to the December 2015 guidance:
- For male donors who would have been deferred for having sex with another man: the agency is changing the recommended deferral period from 12 months to 3 months.
- For female donors who would have been deferred for having sex with a man who had sex with another man: the agency is changing the recommended deferral period from 12 months to 3 months.
- For those with recent tattoos and piercings: the agency is changing the recommended deferral period from 12 months to 3 months.
Revised Recommendations to Reduce the Risk of Transfusion-Transmitted Malaria
The FDA is making the following changes, for immediate implementation, to the August 2013 guidance:
- For those who have traveled to malaria-endemic areas (and are residents of malaria non-endemic countries): the agency is changing the recommended deferral period from 12 months to 3 months. In addition, the guidance provides notice of an alternate procedure that permits the collection of blood and blood components from such donors without a deferral period, provided the blood components are pathogen-reduced using an FDA-approved pathogen reduction device.
The FDA is finalizing the January 2020 draft guidance, which includes the following change from the previous guidance:
- For those who spent time in certain European countries or on military bases in Europe who were previously considered to have been exposed to a potential risk of transmission of Creutzfeldt-Jakob Disease or Variant Creutzfeldt-Jakob Disease, the agency is eliminating the recommended deferrals and is recommending allowing reentry of these donors.
To help address this critical need, the FDA is also providing notice of alternatives to certain requirements regarding blood donor eligibility for the duration of the COVID-19 pandemic. Blood establishments are not required to implement the changes in the FDA recommendations or the alternative procedures.
We expect that the updated guidance and alternative procedures will help increase the number of donations moving forward, while helping to ensure adequate protections for donor health and maintaining a safe blood supply for patients.
We believe these updated recommendations will have a significant and positive impact on our blood supply. As noted above, the changes being announced to the HIV, vCJD and malaria guidances are being put forth for immediate implementation. The updated recommendations in these guidances are based on data and analysis that the FDA believes are applicable to circumstances outside of (and after) the COVID-19 pandemic and reflect the agency’s current thinking on this issue. These recommendations are expected to remain in place after the COVID-19 pandemic ends, with any appropriate changes based on comments we receive and our experience implementing the guidances.
The FDA will provide notification when the alternative procedures are no longer in effect. The FDA will monitor these changes in policy, alongside the National Institutes of Health’s National Heart, Lung and Blood Institute and major blood partners to ensure the continued safety of the blood supply.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.




























 PopUp in the Plaza: Art and Gardening Come Alive in Downtown Rockwall for Founder’s Day Celebration
PopUp in the Plaza: Art and Gardening Come Alive in Downtown Rockwall for Founder’s Day Celebration
